Explaining iron oxide
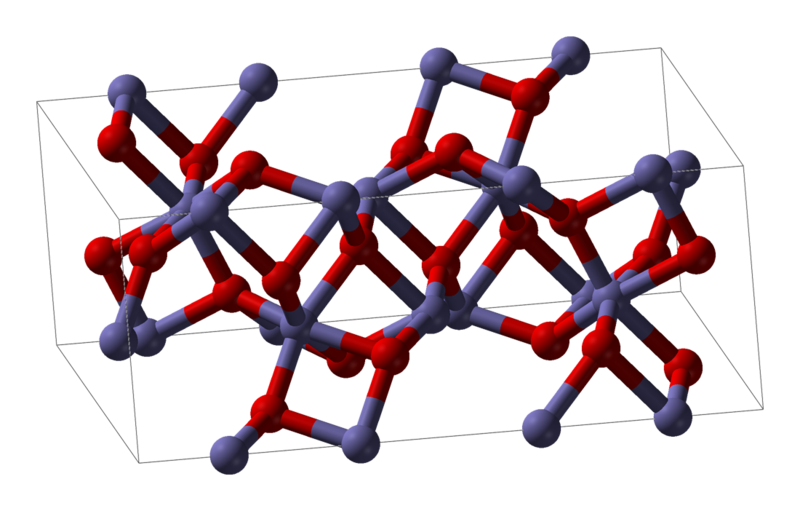
Here is a picture of Fe3O2

To explain this, we look at a model of iron in the cubic atomic model:
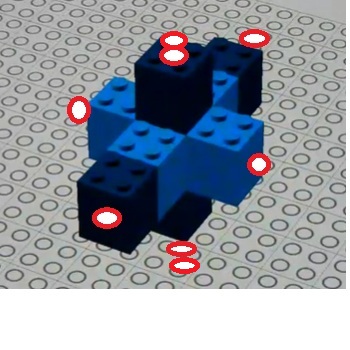
The red circles show where electrons in the form of deutrium particles have been added to argon.
This shows 6 possible exterior bonding points and naturally forms octahedral molecules.
One would predict that 6 bonding sites would be the maximum. This is confirmed by VSEPR charts.
http://qsib.files.wordpress.com/2010/02/vsepr_geometries1.png
However, this is not confirmed by electron shell theory. The electron shell for iron should be:
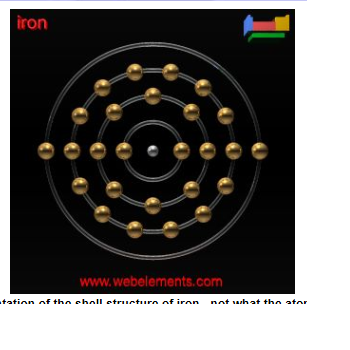
So what's wrong with this picture? It shows that the outside shell which theoretically are the electrons used for binding, only has 2.
So, iron should only form compounds with 2 elements maximum. Instead, we see iron forming bonds with upto 6 atoms in iron oxide.
So how does the electron orbital theory handle this? They use magical hybridization. Need more orbitals, just pull as many as you need out of the lower shell.
Go ahead, help yourself to as many as you need. Of course, this is a big, unjustifiable Ad Hoc kludge. Why can we only pull up the 4 extra we need, why not 6 or 10?
Let's look at the model of oxygen:

The orange circles show the main bonding sites and oxygen generally only takes 2 other atoms.
However, we do see atoms in the iron oxide where the oxygen atoms (in red) appear to take up to 3 bonds.
These bonds appear to form at right angles and would appear that the flat central section (pointing at you in the picture) can also serve as a bonding surface.
This is a bit Ad Hoc, but I don't think as bad as VESPR hybridization where you can just pull orbitals where ever you need them.
So if you press an iron binding site right into the flat part of oxygen, you might get a bond. I would predict it would be weaker than the other 2, but it could happen.
With all of this in mind, we can easily see that iron oxide forms the complex structure whereby the iron atom (in gray) has upto 6 oxygen (in red) partners.
We see that the iron geometry is generally octahedral and the geometry of the oxygen is 2 right angled bonds (x,y direction), plus one bond in the z direction.
So they you have it, iron oxide completely explained without the use of any electron orbitals. In fact it explains it better since electron orbitals should only have iron combining with 2 oxygen.